Abstract
Objective: Acute myeloid leukemia (AML) is the most common indication for alloHCT in the United States (US) for Medicare beneficiaries. An increasing number of older patients are now able to undergo alloHCT; however, there is a significant gap in knowledge about their healthcare costs. The primary objective of this study was to assess healthcare reimbursement, service utilization, and overall survival for designated time points up to 1-year post-alloHCT for Medicare beneficiaries ages 65 and older in the US with an AML diagnosis.
Methods: Medicare claims data from 2010 - 2012 were merged with Center for International Blood & Marrow Transplant Research (CIBMTR) clinical outcomes data. Patients were excluded who underwent alloHCT before 3/1/10 or after 12/31/11 (to allow for 2 months prior and 1-year follow-up), had a prior autologous or alloHCT, or were not enrolled in both Parts A and B 60 days prior to and on date of HCT. The total amount of reimbursement for 1-year post-transplant included the Medicare payment, secondary payer payments, and coinsurance and out-of-pocket payments made by beneficiaries. Cumulative reimbursement for Part A facility inpatient services, outpatient services, home health and hospice services and Part B physician services were inflation-adjusted to 2017 dollars using Centers for Medicare and Medicaid Services (CMS) Market Baskets. Weighted generalized linear models were used to identify key drivers of total reimbursement adjusting for age, gender, transplant center region, year of transplant, Karnofsky score, disease status, donor type, graft type, Sorror comorbidity score, cytomegalovirus (CMV) serostatus, conditioning intensity, graft-versus-host disease (GVHD) prophylaxis, anti-thymocyte globulin (ATG)/Alemtuzumab (Campath) use, and 2-month total reimbursement prior to the index HCT hospitalization or outpatient HCT date. Service utilization was measured by counts of reported claims and total service days. The Kaplan-Meier method was used to estimate the probability of overall survival (OS) stratified by disease status and conditioning intensity.
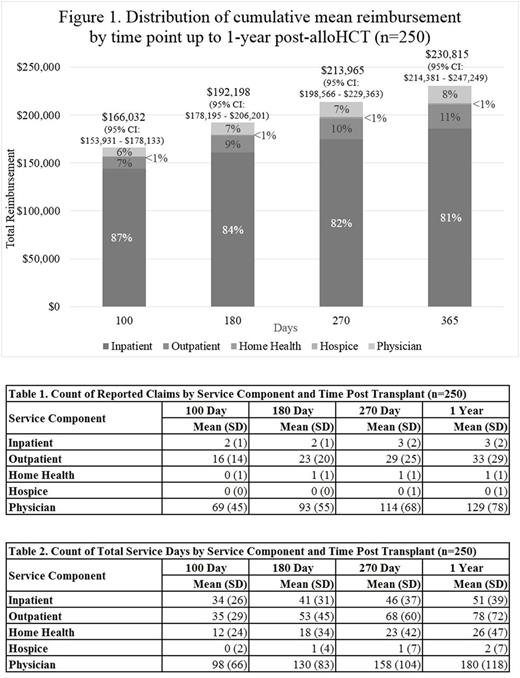
Results: A total of 250 patients met inclusion criteria. Mean age at HCT was 68.7 yrs. The total cumulative reimbursement for the entire cohort were $166,032 (95% confidence interval (CI): $153,931, $178,133) at 100-days and $230,815 (95% CI: $214,381, $247,249) at 1-year post-alloHCT with inpatient reimbursement accounting for more than 80% of the total at each designated time point (Figure 1). The cumulative total reimbursements were $148,684 (95% CI: $137,994, $159,375) for patients surviving 100 days and $186,254 (95% CI: $166,765, $205,744) for patients surviving 1-year post-alloHCT. Counts of claims and total service days by service component and time point post transplant are shown (Tables 1 and 2). In multivariate analysis, patients who received cord blood compared to peripheral blood stem cell had significantly higher adjusted reimbursement (p=0.006). Similarly, myeloablative (MA) conditioning was associated with significantly higher reimbursement compared to non-myeloablative (NMA) (P < 0.0001). The Northeast (p=0.0275) and West (p=0.0323) regions had significantly higher reimbursement compared to the Midwest region. OS at 1-year post-alloHCT was 52% (95% CI: 46%-59%). Patients undergoing alloHCT in CR1/CR2 had better survival (p=0.0030) as did those who received NMA conditioning (P=0.0067).
Conclusions: This unique dataset facilitated an analysis of key drivers of Medicare reimbursement for AML by patient and HCT related variables and outcomes. It was not surprising that a cord blood graft was a driver of reimbursement, likely due to acquisition costs, prolonged time to engraftment and subsequent hospitalization. The variation in reimbursement by region may be partly due to geographic adjustment of Medicare payments or transplant center billing practices. Interestingly, NMA conditioning not only led to lower 1-year reimbursement but also improved survival and may inform clinical practice. Future research will stratify each component of post-transplant reimbursement into specific cost categories to evaluate Medicare reimbursement and effects of practice patterns.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal